OUR PROJECTS

This project fosters a Consortium with support from multiple partners whose goal is to expand dementia research in Latin America and the Caribbean (LAC). The Consortium aims to combine genomic, neuroimaging and behavioral (clinical, cognitive, socioeconomic) data to improve dementia characterization and identify novel inroads to treat neurodegeneration in diverse populations. This 5-year project constitutes an unprecedented opportunity to foster regional synergy and multidisciplinary research to promote harmonization of global strategies to treat and, ultimately, prevent dementia in diverse and underserved populations around the world. We will develop an innovative, harmonized, and cross-regional approach on two of their most prevalent neurodegenerative disorders: Alzheimer’s disease (AD) and frontotemporal dementia (FTD). We combine a R01 funding (“US-South American initiative for genetic-neural-behavioral interactions in human neurodegenerative research”, R01 AG057234) with additional support. The R01 project will provide a basic platform anchored in Argentina, Brazil, Colombia, and Peru, that is supplemented with clinical research expertise from the University of California, San Francisco (UCSF) Memory and Aging Center, genomics expertise from the University of California, Santa Barbara, and bioinformatics infrastructure and expertise from Hudson Alpha. This proposal is extended to collaborators in Mexico and Chile. We also will assess novel families across LAC via the Latin America and Caribbean consortium on Dementia (LAC-CD). In addition to the R01 strategy based on patients with familial and sporadic presentations tested for genetic risks (risk scores), it would also support recruitment of AD and FTD families with an autosomal dominant-like presentation from the LAC-CD. This platform will facilitate numerous research projects from additional groups of the LAC-CD; leverage regional expertise in aging and dementia to establish a multidisciplinary, collaborative consortium of diverse, international clinicians and researchers; and promote the development of the next generation of aging and dementia investigators in LAC. In this expanded framework, we would first screen all patients for known AD/FTD/ALS genes and then, for those who screen negative for known genetic causes of disease, we will perform whole-genome sequencing (WGS) for gene discovery. By leveraging the R01 efforts and extending the project scope to include Mexico, Chile, and novel families from LAC-CD, we will establish a network of AD and FTD families and clinicians/researchers, enabling large-scale research to identify novel genetic and SES contributions to AD and FTD in diverse populations. By establishing this collaborative framework, which capitalizes on unique regional populations, our proposal can consolidate a LAC-based platform for future translational research and assessment. Our long-term goal is to identify the unique genetic and SES factors that drive AD and FTD presentation in LAC relative to the US, including risk factors, cognitive profiles and brain imaging. To this end, we will establish a first-in-class cohort anchored in six LAC (Argentina, Chile, Colombia, Brazil, Mexico, and Peru), compared to US samples (totaling > 4200 participants, including 2100 controls, 1050 AD patients, and 1050 FTD patients), led by world-renowned leaders in dementia research. We will couple standardized clinical assessments with innovative analytical techniques to account for heterogeneity in these diverse populations. By combining standardized genetic, neuroimaging, and behavioral (cognitive and SES) measures, we will test the underlying hypothesis that there are unique risk factors for AD and FTD in LAC (e.g., genetic risk factors enriched in LAC populations; underlying cognitive and neural vulnerability due to SES) compared to US populations. Our plan to recruit large numbers of controls and patients across these diverse populations will provide excellent opportunities to identify new genetic and SES risks for AD and FTD. In addition, the machine learning strategies we have developed to reduce the impact of background heterogeneity will allow us to refine the accuracy of our association studies. In this context, we will pursue the following Specific Aims:
Aim 1: To establish genetic contributions to AD and FTD in diverse LAC cohorts (Tier 1 study, with larger sample size than Tier 2). By elucidating the genetic substructure and familial contributions to AD and FTD in LAC relative to the US, we will be able to identify proper populations for replication of our genetic findings. By assembling this large cohort, we will also be well positioned to establish a LAC-specific polygenic risk score (PRS) for predicting AD and FTD risk in future samples.
- To identify the prevalence of autosomal dominant and rare variant risk factors for AD and FTD in target genes. We hypothesize that, relative to the US, LAC have a higher frequency of familial forms of AD and FTD. Discovery of new families with multiple affected individuals will advance efforts to treat AD and FTD in patients with rare mutations (PS1, APP, MAPT, GRN, and C9orf72).
- To perform exploratory genome-wide association studies (GWAS) in LAC subpopulations. We hypothesize that, relative to US patients, LAC are enriched for novel risk in genes for lipid metabolism (AD) and lysosomal function (FTD).
- To test whether PRS developed in European AD and FTD populations prove valid in LAC. We hypothesize that PRS will work best at discriminating patients from controls in the European predominant subpopulation (US and, to a lesser extent, Argentina, Chile) than in the African and Indigenous-majority admixed cohorts (Peru, Brazil, Colombia, Mexico).

Aim 2: To elucidate the impact of SES on clinical, cognitive, and brain imaging signatures in LAC and the US. (Tier 2 study-comprehensive imaging and cognitive evaluation in a subset of Tier 1). To compare patients across regions, we need to establish standardized neurocognitive measures and understand how SES impacts the manifestations of dementia in LAC.
- To evaluate how SES moderates the relationship between age at onset and disease severity in AD and FTD. We hypothesize that AD and FTD will emerge at an earlier age in low-SES vs. high-SES (dichotomized) patients, and measures of disease severity, including cognitive performance, and multimodal neuroimaging, will be worse in the low-SES group even after accounting for age.
- To assess the differential impact of SES on clinical, cognitive and brain imaging deficits in LAC vs. US samples. We hypothesize that difference in disease severity ratings, cognition, and multimodal neuroimaging that reflect low vs. high SES disparities will be greater in LAC patients compared to US patients.
Aim 3: To determine whether genetic risk and SES yield better discrimination between LAC and US patients as compared with other cognitive, neuroimaging, and clinical variables (Tier 1 & 2). To date, no study has sought to establish which potential predictors prove more sensitive to discriminate between LAC and US patients. In particular, although genetic risk and SES (Aims 1 and 2) have the potential to robustly differentiate between such samples, no study has explored their role, let alone as compared to other multimodal factors. To address this issue, we will apply data-driven machine-learning analysis to determine top factors that best discriminate patients in LAC from those in the US. Multimodal measures from controls of each country will be used for population-specific normalization of patient data. We hypothesize that the top features, better discriminating LAC from US patients will be related to SES and genetic risk (e.g., standardized PRS) in comparison to other variables (clinical, cognitive, and imaging measures).
The expected outcome of this study is a large Latin American cohort of harmonized, well-characterized AD and FTD patients and controls (Fig 1). Positive impacts of this work include a better understanding of genetic and SES contributions to neurocognitive manifestations of dementia and identification of novel targets for risk reduction and disease prevention in LAC. Our large multimodal, cross-sectional study will enable clinical assessment of understudied patient groups, extend and harmonize existing data sets, prompt the development of novel measures, and inform future work on the clinical value of combined multimodal profiles to predict disease presentation and progression in longitudinal studies of diverse populations.

To further our understanding of the inner workings of the human brain, we need to look at this organ as a networks framework. This approach is becoming more feasible with the advent of technologies which are enabling the integration of multiple methodologies to disentangle the functional architecture of the human brain. In this endeavour, it is important to learn about healthy brains and also about brains undergoing pathological changes as both approaches will offer valuable insights into the vulnerability, or lack thereof, of the individual nervous system. Thanks to the support from the Medical Research Council - UK, we have set up a collaboration network which aims to address these challenges using state of the art methodologies in neuroscience.
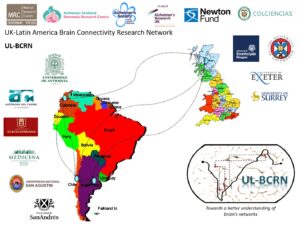
We have called this network UK-Latin America Brain Connectivity Research Network. Its target is to capitalise on existing resources at the member laboratories as well as on the experience accrued by these groups over many years of work with the vision to forge a close relationship leading to the consolidation of a shared methodological context. This network will allow access to unique populations of individuals who suffer from rare forms of neurodegenerative diseases, will permit assessment of hypotheses across cultures and ethnicities, and will promote training of new researchers globally. We envisage that all these opportunities will not only lead to major contributions to the field but will do that in a timely manner.
If you are interested, please fill in the following form:
Publications
Ibañez, A., & Parra, M. A. (2014). Mapping memory binding onto the connectome's temporal dynamics: toward a combined biomarker for Alzheimer's disease. Front Hum. Neurosci, 8, 237. doi:10.3389/fnhum.2014.00237 [doi]
Parra, M. A., Ascencio, L. L., Urquina, H. F., Manes, F., & Ibanez, A. M. (2012). P300 and neuropsychological assessment in mild cognitive impairment and Alzheimer dementia. Front Neurol, 3, 172. doi:10.3389/fneur.2012.00172 [doi]
Parra, M. A., Mikulan, E., Trujillo, N., Della Sala, S., Lopera, F., Manes, F., . . . Ibáñez, A. (2017). Brain information sharing during visual short-term memory binding yields a memory biomarker for familial Alzheimer's disease. Current Alzheimer Research, 14(12), 1335-1347.
Pietto, M., Parra, M. A., Trujillo, N., Flores, F., Garcia, A. M., Bustin, J., . . . Baez, S. (2016). Behavioral and Electrophysiological Correlates of Memory Binding Deficits in Patients at Different Risk Levels for Alzheimer's Disease. J Alzheimers Dis, 53(4), 1325-1340. doi:JAD160056 [pii];10.3233/JAD-160056 [doi]
Smith, K., Ricaud, B., Shahid, N., Rhodes, S., Starr, J. M., Ibanez, A., . . . Vandergheynst, P. (2017). Locating Temporal Functional Dynamics of Visual Short-Term Memory Binding using Graph Modular Dirichlet Energy. Scientific Reports, 7, 42013. doi:Article
Spyrou, L., Parra, M., & Escudero, J. (2018). Complex tensor factorisation with PARAFAC2 for the estimation of brain connectivity from the EEG. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 1-1. doi:10.1109/TNSRE.2018.2883514
Quintero-Zea, A., López, J. D., Smith, K., Trujillo, N., Parra, M. A., & Escudero, J. (2018). Phenotyping Ex-Combatants From EEG Scalp Connectivity. IEEE Access, 6, 55090-55098. doi:10.1109/ACCESS.2018.2872765
Trujillo, S. P., Valencia, S., Trujillo, N., Ugarriza, J. E., Rodriguez, M. V., Rendon, J., . . . Parra, M. A. (2017). Atypical Modulations of N170 Component during Emotional Processing and Their Links to Social Behaviors in Ex-combatants. Front Hum. Neurosci, 11, 244. doi:10.3389/fnhum.2017.00244 [doi]
